Multiple strengths to fit your patients' needs

Wide range of dosage options1
- 5 dosage strengths provide the opportunity for single-vial dosing
- Each dose is color-coded
- Each kit contains 5 mL of Sterile Water for Injection and a BAXJECT® II transfer device
Initial dose for control and prevention of bleeding episodes and perioperative management1
Patients ≥12 years of age
An empirical estimate for incremental recovery of 0.9 IU/dL of plasma (0.9% of normal) is used to calculate the initial dose of RIXUBIS with the following formula:

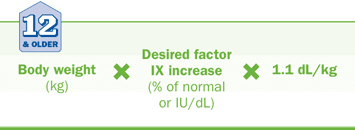
Patients <12 years of age
An empirical estimate for incremental recovery of 0.7 IU/dL of plasma (0.7% of normal) is used to calculate the initial dose of RIXUBIS with the following formula:


Please see RIXUBIS full Prescribing Information for complete dosing guidelines and example calculations.
Twice-weekly dosing for routine prophylactic treatment1
| Dose | Dosing Frequency | |
|---|---|---|
| PTPs ≥12 years of age | 40‑60 IU/kg | Twice‑weekly |
| PTPs <12 years of age | 60‑80 IU/kg | Twice‑weekly |

Adjust the dose based on the individual patient’s age, bleeding pattern, and physical activity.
Dosing for on-demand treatment and control of bleeding episodes1
Type of Bleeding Episodes
Uncomplicated hemarthrosis, superficial muscular or soft tissue
Circulating Factor IX Level Required (% or IU/dl)
Dosing Interval (hours)
Duration of Therapy (days)
Intramuscular or soft tissue with dissection, mucous membranes, hematuria
Circulating Factor IX Level Required (% or IU/dl)
Dosing Interval (hours)
Duration of Therapy (days)
Pharyngeal, retropharyngeal, retroperitoneal, CNS
Circulating Factor IX Level Required (% or IU/dl)
Dosing Interval (hours)
Duration of Therapy (days)
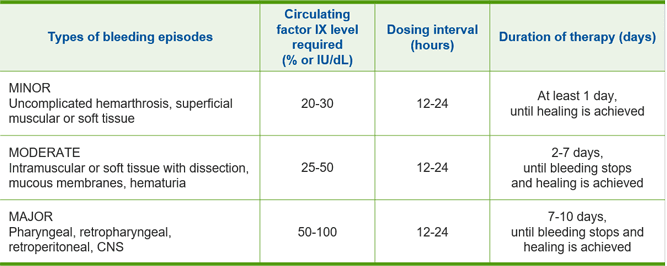
Ensure the factor IX activity level is achieved and maintained in the corresponding period.
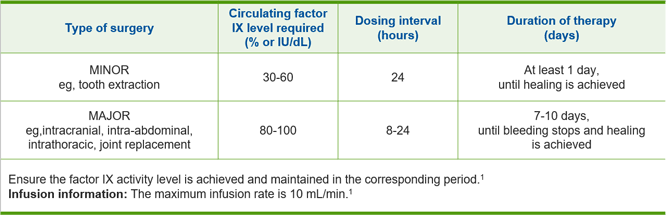
Dosing for perioperative management1
Type of Surgery
e.g., tooth extraction
Circulating Factor IX Level Required (% or IU/dl)
Dosing Interval (hours)
Duration of Therapy (days)
e.g., intracranial, intraabdominal, intrathoracic, joint replacement
Circulating Factor IX Level Required (% or IU/dl)
Dosing Interval (hours)
Duration of Therapy (days)
Mixing is facilitated by the BAXJECT® II Needle-less Transfer device1
The BAXJECT II is needle-less and has a one-piece design.1

For step-by-step instructions on reconstituting RIXUBIS with the BAXJECT II Needle-less Transfer device, watch the step-by-step video. You may also download and read these instructions if your patients are ready to start reconstituting or experience difficulty with the BAXJECT II device.
How to store RIXUBIS®1
Store at refrigerated temperature [2°C to 8°C (36°F to 46°F)] or store at room temperature [not to exceed 30°C (86°F)] for up to 36 months. Do not freeze.
Do not use beyond the expiration date printed on the carton or vial.
![RIXUBIS [Coagulation Factor IX (Recombinant)]](/dist/images/Rixubis-header-logo.png)