2x weekly prophylactic dosing schedule can be adjusted to align factor levels with the times your patients are physically active1
Efficacy
Study design ≥12 years of age
The efficacy and safety of RIXUBIS® were evaluated in a prospective, open-label, uncontrolled, multicenter study of 56 previously treated patients (PTPs) with severe or moderately severe hemophilia B who received RIXUBIS either for routine prophylaxis (mean duration 6.19 months) or on-demand treatment.1,6
Study design <12 years of age
In the pediatric study, the efficacy and safety of RIXUBIS were evaluated in 23 previously treated patients (PTPs) with severe or moderately severe hemophilia B. Patients between 1.8 and 11.8 years of age received RIXUBIS for routine prophylaxis and control of bleeding episodes for a mean treatment duration of 7.7 months.1,2
Selected Important Risk Information for RIXUBIS [Coagulation Factor IX (Recombinant)]
CONTRAINDICATIONS
RIXUBIS is contraindicated in patients who have:
- Known hypersensitivity to RIXUBIS or its excipients including hamster protein
- Disseminated Intravascular Coagulation (DIC)
- Signs of fibrinolysis
ADVERSE REACTIONS
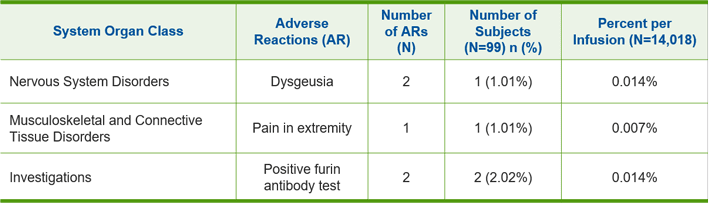
Common adverse reactions observed in >1% of subjects in clinical studies were: dysgeusia, pain in extremity, and positive test for furin antibody.
Routine prophylactic treatment helped patients achieve a low annualized bleed rate (ABR)*1
(range: 0.0-23.4 for PTPs ≥12 years of age and 0-10.8 for PTPs <12 years of age)

Median ABR (range: 0.0-15.6)
Median ABR (range: 0.0-21.5)

Median ABR (range: 0.0-2.0)
Median ABR (range: 0.0-7.2)
*The majority of patients ≥12 years taking RIXUBIS had joint disease (88%) and target joints (66%) at screening.
The efficacy and safety of RIXUBIS® [Coagulation Factor IX (Recombinant)] were evaluated in a prospective, open-label, uncontrolled, multicenter study of 73 male PTPs between 12 and 65 years of age who received RIXUBIS either for routine prophylaxis or on-demand treatment for a median of 156 exposure days for a total of 249 bleeds. The efficacy and safety of RIXUBIS were evaluated in a clinical study, in which a total of 23 male PTPs between 1.8 and 11.8 years of age (median age 7.10 years), with 11 subjects <6 years of age, received RIXUBIS for routine prophylaxis and control of bleeding episodes for a minimum of 150 exposure days for a total of 26 bleeds.
With RIXUBIS, 96.2% of bleeding episodes were rated as having excellent or good bleed resolution in both children and adults.*1
*Excellent is defined as full relief of pain and cessation of objective signs of bleeding after a single infusion; no additional infusion was required for the control of bleeding. Good is defined as definite pain relief and/or improvement in signs of bleeding after a single infusion; possibly requires more than one infusion for complete resolution.
Clinical trials demonstrated that recovery levels were consistent over time1
Effectively resolved most bleeds with 1 to 2 infusions1


RIXUBIS® safety profile
During clinical development in a combined study, 99 male PTPs received a total of 14,018 RIXUBIS infusions and were treated with RIXUBIS for a median of 156 exposure days (range: 8 to 316 days), with a median number of 163 infusions (range: 8 to 327 infusions).1
A total of 337 adverse events were reported in 80 (80.8%) of the 99 subjects. Adverse reactions that occurred in >1% of subjects are shown in the table below:
Summary of Adverse Reactions
No subjects developed neutralizing antibodies to factor IX. Low-titer, non-neutralizing antibodies to factor IX were observed in 21 (21.2%) subjects at 1 or more time points. No clinical adverse findings were observed in any of these 21 subjects.
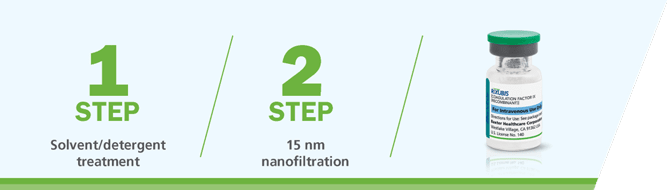
RIXUBIS is manufactured using a validated 2-step virus inactivation and removal process1
Takeda has over 70 years of dedicated history to the hematology and rare disorders communities.11 RIXUBIS® is a third-generation recombinant factor IX, produced by DNA technology. No human or animal proteins are added during any stage of manufacturing or formulation of RIXUBIS.1
Selected Important Risk Information for RIXUBIS [Coagulation Factor IX (Recombinant)]
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions have been reported with RIXUBIS. Anaphylaxis and other hypersensitivity reactions are possible. The risk is highest during the early phases of initial exposure in previously untreated patients (PUPs), in particular in patients with high-risk gene mutations. Early signs of allergic reactions, which can progress to anaphylaxis, include angioedema, chest tightness, hypotension, lethargy, nausea, vomiting, paresthesia, restlessness, wheezing, and dyspnea. Should symptoms occur, immediately discontinue RIXUBIS and administer appropriate treatment.
Inhibitors
Development of neutralizing antibodies (inhibitors) to RIXUBIS may occur. Regularly evaluate patients for the development of factor IX inhibitors by appropriate clinical observations and laboratory tests. If expected factor IX plasma activity levels are not attained, or if bleeding is not controlled with an expected dose, perform an assay that measures factor IX inhibitor concentration. Contact a specialized hemophilia treatment center if a patient develops an inhibitor. Patients with factor IX inhibitors are at an increased risk of severe hypersensitivity reactions or anaphylaxis if re-exposed to RIXUBIS.
![RIXUBIS [Coagulation Factor IX (Recombinant)]](/dist/images/Rixubis-header-logo.png)



